Brand-name and generic Suboxone contain identical amounts of buprenorphine and naloxone, delivering the same therapeutic benefits for opioid dependence treatment. You’ll find both versions as sublingual films in matching strengths (2mg/0.5mg to 12mg/3mg). The key difference lies in cost: generic versions can save you over $1,400 annually while maintaining FDA-approved safety standards. Both feature citrus flavoring and similar dissolution times. Understanding these options helps you make informed decisions about your treatment [path].
Active Ingredients and Drug Composition

When comparing brand-name Suboxone with its generic counterparts, you’ll find identical active ingredients at their core. Both formulations contain the same amounts of buprenorphine and naloxone, operating through an identical mechanism of action to treat opioid dependence.
Brand and generic Suboxone contain identical buprenorphine and naloxone levels, working the same way to treat opioid dependence.
Under strict regulatory oversight, these medications work as partial agonist-antagonist combinations. The buprenorphine component provides consistent therapeutic effects, while naloxone prevents misuse by triggering withdrawal if injected. The sublingual film formulation dissolves effectively in the mouth while keeping the naloxone component inactive. Brand-name Suboxone is distinctly recognizable by its orange packaging and citrus flavor.
Despite significant pricing trends showing cost differences between brand and generic versions, their fundamental drug composition remains unchanged. A typical 30-day supply cost ranges from $40 for generic to $150 for brand-name versions.
You can be certain that whether choosing brand-name or generic Suboxone, you’re getting the same active components working in the same way. The FDA guarantees both versions maintain identical therapeutic profiles for treating opioid dependence effectively.
Available Forms and Dosage Strengths
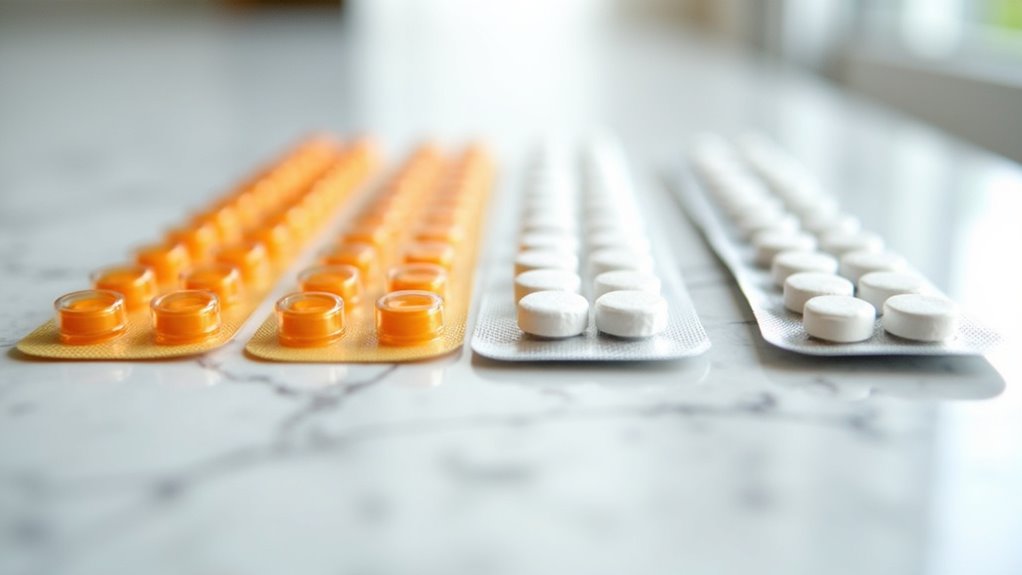
You’ll find that both brand-name Suboxone and its generic alternatives are exclusively available as sublingual films, with tablets having been discontinued since 2010. The films come in identical dosage strengths of 2mg/0.5mg, 4mg/1mg, 8mg/2mg, and 12mg/3mg buprenorphine/naloxone combinations across all manufacturers.
These standardized formulations guarantee you’re receiving therapeutically equivalent treatment whether you choose brand or generic versions, as the FDA requires strict adherence to these established dosage parameters. Both versions deliver equally safe and effective treatment outcomes, though generic options typically cost less due to reduced testing and marketing expenses. Many patients find that mail-order pharmacies provide a convenient and cost-effective way to receive their medication. Patients should be aware that currently no generic formulation of the sublingual tablet version is available on the market.
Film Vs Tablet Options
Suboxone offers two distinct formulations: sublingual films (strips) and tablets, each available in diverse dosage strengths for treating opioid use disorder. When choosing between options, you’ll notice significant differences in administration and patient preferences. The cost difference between formulations can be substantial, with tablets typically being more affordable. Recent studies show that patients taking sublingual films should exercise extra caution as dental issues have been reported. The dissolution process takes significantly longer with tablets, as they require up to 12.4 minutes to fully dissolve.
| Feature | Films/Strips | Tablets |
|---|---|---|
| Absorption Rate | Faster dissolution | Slower GI absorption |
| Administration | Under tongue, no water needed | Requires water to swallow |
| Taste/Texture | Citrus flavored | Neutral taste |
| Misuse Risk | Lower, can’t be crushed | Higher potential for alteration |
The strips demonstrate superior bioavailability and quicker absorption compared to tablets, while offering bolstered protection against misuse. Patient preferences often favor strips due to their sensory factors, including citrus flavor and rapid dissolution. Clinical evidence supports equivalent efficacy between both forms, though you may need dose adjustments when switching between formulations due to bioavailability differences.
Matching Dosage Strengths Available
Building on the discussion of formulation types, understanding the available dosage strengths of brand and generic Suboxone helps guarantee proper therapeutic management.
You’ll find that generic Suboxone matches the brand’s four primary strengths: 2mg/0.5mg, 4mg/1mg, 8mg/2mg, and 12mg/3mg formulations. Each formulation contains lactose and mannitol as key inactive ingredients. These generics meet strict bioequivalence standards and carry an “AB” rating, confirming their interchangeability with brand Suboxone. Healthcare providers can adjust dosages progressively in increments or decrements to achieve optimal therapeutic effects. However, certain brand-specific strengths remain exclusive, such as Cassipa’s 16mg/4mg formulation and Zubsolv’s unique dosing range. The typical maintenance phase requires 4 mg to 24 mg of buprenorphine for optimal treatment outcomes.
When switching between brand and generic Suboxone films, no dosage conversion protocols are necessary. However, if you’re changing or shifting patients from other branded products like Zubsolv or Bunavail, you’ll need to adjust dosages due to their distinct formulation strengths and absorption profiles.
Treatment Costs and Insurance Coverage
Understanding your insurance coverage for Suboxone treatment is pivotal, as costs can vary dramatically between brand-name and generic options.
Most patients find relief knowing that Medicare and Medicaid typically provide coverage for their prescriptions.
You’ll find brand Suboxone films typically cost $170-$270 for a 30-day supply, while generic versions start markedly lower at $35-$94, representing potential annual savings of over $1,400.
Your out-of-pocket expenses will depend heavily on your insurance plan’s formulary placement, with many insurers requiring prior authorization for brand Suboxone while offering more straightforward access to generics. Some patients may find that using pharmacy coupons provides even better savings than their insurance coverage.
Eligible patients can utilize patient assistance programs that offer cost-free or significantly discounted medications if they meet specific income and insurance criteria.
Insurance Coverage Landscape
While maneuvering treatment options for opioid dependency, insurance coverage plays an essential role in determining access to both brand-name and generic Suboxone. You’ll find that Medicare Part D and most ACA-compliant plans cover these medications, though prior authorization requirements and quantity limit policies often apply.
Coverage specifics vary considerably between insurers, with generic versions typically receiving more favorable formulary placement and lower cost-sharing structures.
Key insurance considerations for Suboxone coverage:
- Medicare Part D plans may cover both formulations, but generics often have fewer restrictions
- Prior authorization is more commonly required for brand-name versions
- Generic versions typically fall into lower copay tiers ($10-$30) versus brand-name ($30-$75)
- Annual formulary updates can affect coverage status and tier placement
- Manufacturer savings cards can help offset brand-name costs up to $75 monthly
Monthly Cost Comparison
The stark cost differential between brand-name and generic Suboxone creates significant financial implications for patients seeking opioid dependency treatment. You’ll find brand Suboxone strips (8mg/2mg) costing around $10 daily, totaling $300 monthly, while higher doses (16mg/4mg) can reach $600 monthly in out-of-pocket costs.
Generic alternatives offer substantial savings, with tablets ranging from $3-$8 per unit, potentially reducing monthly expenses by 50% or more. Payment assistance programs and pharmacy comparison tools like GoodRx can further decrease costs to approximately $35.44 monthly. If you’re uninsured, transparent cash pricing options exist at some pharmacies, offering treatment for $195 monthly. Your choice between strips and tablets impacts both cost and treatment adherence, with brand strips typically commanding premium pricing for their convenience.
Safety and Clinical Effectiveness
As research consistently demonstrates, brand-name Suboxone and its generic counterparts maintain equivalent safety profiles and clinical effectiveness when used for opioid dependence treatment. However, dental health considerations differ between formulations, with film versions showing higher oral exposure risks compared to tablet forms.
While brand-name and generic Suboxone work equally well, tablet forms may offer better dental protection than film versions during treatment.
Both options deliver identical active ingredients and undergo rigorous FDA testing to guarantee bioequivalence and consistent quality standards.
- Both formulations effectively manage withdrawal symptoms and cravings
- Generic tablets may reduce dental risks associated with prolonged sublingual exposure
- Clinical monitoring requirements remain identical for both versions
- Manufacturing follows strict Good Manufacturing Practices (GMP)
- Adverse reaction patterns show no significant differences between options
You’ll find the same therapeutic benefits regardless of choice, though individual factors like pre-existing dental conditions might influence which form is most appropriate for your treatment plan.
Packaging Features and Flavor Options
Significant differences in packaging and flavor profiles distinguish brand-name Suboxone from its generic counterparts. You’ll notice brand-name Suboxone’s distinctive orange packaging and citrus-flavored films, while generic versions offer varied designs and taste options. These branding considerations impact patient acceptance factors and treatment compliance.
| Feature | Brand vs Generic Comparison |
|---|---|
| Packaging Color | Orange (Brand) vs Different Colors (Generic) |
| Flavor Options | Citrus Only (Brand) vs Mint/Unflavored (Generic) |
| Form Available | Films Only (Brand) vs Films & Tablets (Generic) |
| Logo Presence | Distinctive Branding (Brand) vs Basic Labels (Generic) |
Since 2012, brand-name Suboxone has exclusively offered sublingual films, while generics maintain both film and tablet options. The brand’s consistent citrus flavor serves as a misuse deterrent, whereas generic manufacturers focus on providing diverse taste alternatives to improve patient acceptance.
Storage and Administration Guidelines
Proper storage and administration protocols remain indispensable for maintaining Suboxone’s therapeutic efficacy, whether you’re using brand-name or generic formulations. You’ll need to store your medication at 68-77°F in airtight containers, while following strict handling techniques to prevent accidental ingestion risks. Both films and tablets require sublingual placement, though films typically dissolve faster than their tablet counterparts.
- Store in a locked, temperature-controlled environment (20-25°C)
- Don’t eat or drink for 2 hours before taking your dose
- Place medication under your tongue and allow complete dissolution
- Never chew, swallow, or bend the medication
- Keep medications in their original packaging until use
Remember that proper administration directly impacts treatment success, so it’s essential to follow your healthcare provider’s specific instructions for ideal results.
Market Availability and Manufacturing Standards
Beyond storage protocols, understanding market dynamics and manufacturing standards helps you make informed decisions about Suboxone treatment options. You’ll find generic versions widely available at most pharmacies, while brand-name supplies may fluctuate due to demand. Regional access differences show North America leading in availability, though supplier dynamics are evolving with major manufacturers like Dr. Reddy’s and Mylan driving generic distribution.
Both brand and generic forms must meet strict FDA bioequivalence standards. While inactive ingredients may vary between versions, they uphold therapeutic equivalence through rigorous quality control processes. You’ll notice generics typically cost less due to lower production expenses, yet they follow identical FDA labeling requirements. This standardization guarantees you’re receiving safe, effective treatment regardless of which version you choose.
Frequently Asked Questions
Can I Switch Between Brand and Generic Suboxone During Treatment?
Yes, you can safely switch between brand and generic Suboxone during treatment. Both versions offer equal treatment effectiveness since they contain identical active ingredients and meet FDA bioequivalence standards.
While you might notice slight differences in taste or texture, the therapeutic outcomes remain the same. Consider discussing the move with your healthcare provider, who can monitor your shift and help evaluate cost comparison benefits, especially if insurance coverage changes.
How Long Does It Take to Adjust to Generic Suboxone?
Your adjustment to generic Suboxone typically takes the same amount of time as brand-name versions, usually 3-7 days. Since generics are pharmacologically equivalent, you shouldn’t experience significant differences in medication dosage adjustments.
Your body primarily needs to adapt to the medication itself, not the formulation type. If you’re already stabilized on brand Suboxone, you’ll likely maintain stability when switching to generic, as there aren’t meaningful pharmacological effectiveness differences between them.
Why Do Some Doctors Prefer Prescribing Brand-Name Suboxone Over Generics?
Doctors often prefer prescribing brand-name Suboxone because it shows more consistent pharmacokinetics and reliable absorption rates. You’ll find that brand-name films dissolve more predictably under the tongue, which improves medication access and reduces dosing errors.
While cost considerations favor generics, many physicians prioritize treatment stability and patient compliance. They’ve observed that brand-name versions may offer better therapeutic outcomes, even though insurance coverage and prior authorizations can complicate access.
Are Withdrawal Symptoms Different When Using Generic Versus Brand Suboxone?
Yes, you may experience different withdrawal symptoms when using generic versus brand Suboxone. Research shows potential effectiveness differences, with some patients reporting more intense withdrawal symptoms after switching to generics, including increased cravings, insomnia, and muscle pain.
While both formulations contain identical active ingredients, individual responses vary based on patient preference considerations and metabolism. You’ll want to work closely with your healthcare provider to monitor and adjust dosing if needed during any shift.
Do Pharmacies Always Stock Both Brand and Generic Versions?
No, you won’t find both versions at every pharmacy. Pharmacy inventory levels vary noticeably based on local demand and supply chain factors.
While most pharmacies reliably stock generic versions, brand-name Suboxone availability is less consistent. Despite patient medication preferences, many pharmacies choose to primarily stock generics due to cost efficiency and insurance coverage policies.
You’ll find that large chain pharmacies particularly favor generics, while some smaller pharmacies might maintain limited quantities of both versions.

